- Journal List
- Molecules
- v.16(10); 2011 Oct
- PMC6264685

Silver Nanoparticles as Potential Antiviral Agents
Abstract
Virus infections pose significant global health challenges, especially in view of the fact that the emergence of resistant viral strains and the adverse side effects associated with prolonged use continue to slow down the application of effective antiviral therapies. This makes imperative the need for the development of safe and potent alternatives to conventional antiviral drugs. In the present scenario, nanoscale materials have emerged as novel antiviral agents for the possibilities offered by their unique chemical and physical properties. Silver nanoparticles have mainly been studied for their antimicrobial potential against bacteria, but have also proven to be active against several types of viruses including human imunodeficiency virus, hepatitis B virus, herpes simplex virus, respiratory syncytial virus, and monkey pox virus. The use of metal nanoparticles provides an interesting opportunity for novel antiviral therapies. Since metals may attack a broad range of targets in the virus there is a lower possibility to develop resistance as compared to conventional antivirals. The present review focuses on the development of methods for the production of silver nanoparticles and on their use as antiviral therapeutics against pathogenic viruses.
1. Introduction
Viruses represent one of the leading causes of disease and death worldwide. Thanks to vaccination programmes, some of the numerous diseases that used to kill many and permanently disable others have been eradicated, such as smallpox in 1979 [1] or have greatly reduced the burden of the disease, such as in the case of the paralytic disease poliomyelitis [2]. However, for some of today’s most pressing viral pathogens, there is still no vaccine available. To realize the huge economic impact that several viral diseases cause to the global community, we need only to think to common colds, influenza, various problems due to herpesviruses (from shingles, genital herpes, chickenpox, infectious mononucleosis, up to herpes keratitis, neonatal disseminated infections, or viral encephalitis). Other viruses are also able to cause considerable distress and sometimes persistent infections that may lead to cancer or to acquired immunodeficiencies, such as hepatitis viruses (mainly HBV and HCV) or human immunodeficiency virus (HIV). Much effort has been expended in attempts to develop vaccines for these diseases, without appreciable success, at least for some of these viruses, namely, HCV, HIV and some herpesviruses. Presently, the development of new vaccines for such viruses seems likely to continue to be elusive. Together with the risk of emerging or re-emerging viral agents, the field of antiviral compound discovery is very promising.
Emerging and re-emerging viruses are to be considered a continuing threat to human health because of their amazing ability to adapt to their current host, to switch to a new host and to evolve strategies to escape antiviral measures [3].
Viruses can emerge because of changes in the host, the environment, or the vector, and new pathogenic viruses can arise in humans from existing human viruses or from animal viruses. Several viral diseases that emerged in the last few decades have now become entrenched in human populations worldwide. The best known examples are: SARS coronavirus, West Nile virus, monkey pox virus, Hantavirus, Nipah virus, Hendravirus, Chikungunya virus, and last but not least, the threat of pandemic influenza viruses, most recently of avian or swine origin. Unfortunately the methodological advances that led to their detection have not been matched by equal advances in the ability to prevent or control these diseases. There have been improvements in antiviral therapy, but with a wide margin of ineffectiveness, therefore new antiviral agents are urgently needed to continue the battle between invading viruses and host responses. Technological advances have led to the discovery and characterization of molecules required for viral replication and to the development of antiviral agents to inhibit them. Most viruses are, indeed, provided by an extraordinary genetic adaptability, which has enabled them to escape antiviral inhibition and in certain cases to regain advantage over the host by mutagenesis that create new viral strains with acquired resistance to most of the antiviral compounds available [3].
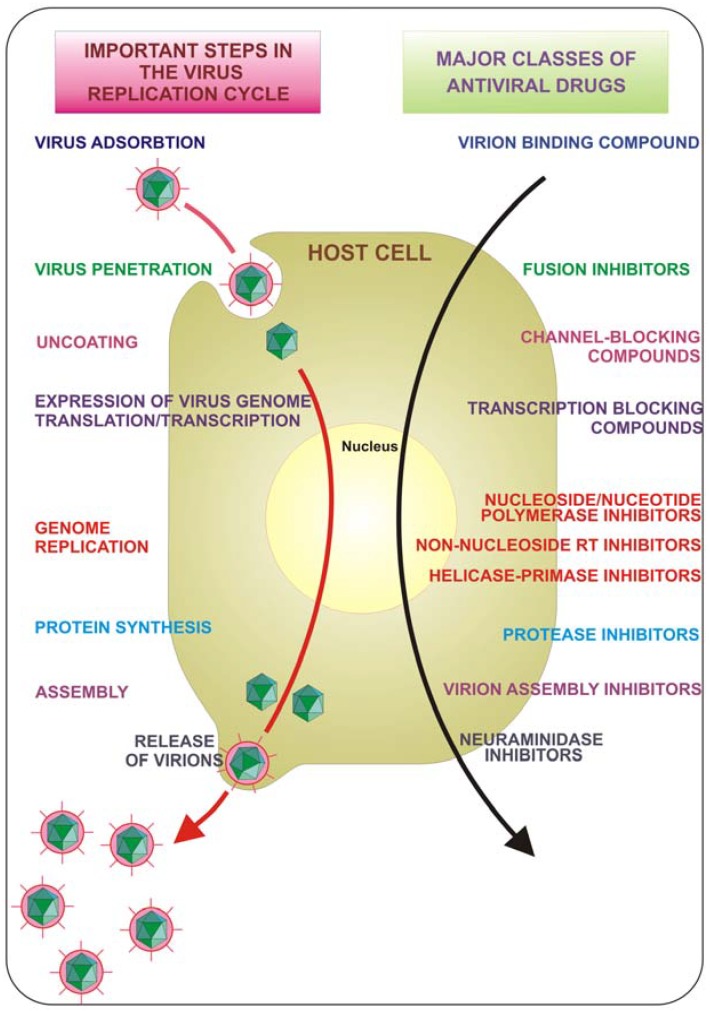
The course of viral infections is governed by complex interactions between the virus and the host cellular system. All viruses depend upon a host cell for their protein synthesis. Thus, all viruses replicate via a broadly similar sequence of events (Figure 1). The virus must first bind to the cell, and then the virus or its genome enters in the cytoplasm. The genome is liberated from the protective capsid and, either in the nucleus or in the cytoplasm, it is transcribed and viral mRNA directs protein synthesis, in a generally well regulated fashion. Finally, the virus undergoes genome replication and together with viral structural proteins assembles new virions which are then released from the cell. Each of the single described phases represents a possible target for inhibition. Drugs that target viral attachment or entrance have proved to be very difficult to be discovered. In fact, to date, only one entry inhibitor has been approved by the US Food and Drug Administration (FDA). This is enfuvirtide (T-20), a synthetic peptide that targets the HIV gp41 envelope protein to prevent fusion.